CDC Director Overrules Panel, Approves Pfizer COVID Booster Shots For Frontline Workers
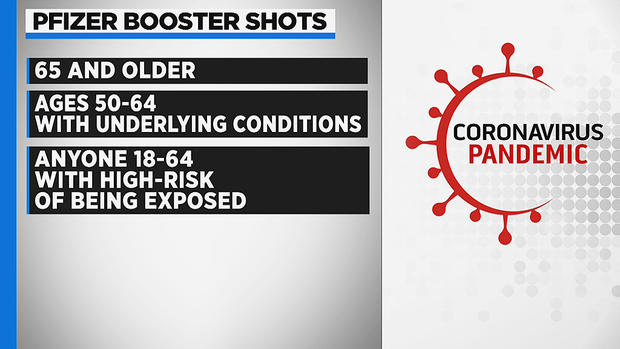
BOSTON (CBS/AP) - Centers for Disease Control and Prevention Director Dr. Rochelle Walensky overruled a panel of advisers and approved a booster shot for people ages 18 to 64 years who are health care workers or have another essential job that puts them at increased risk of being exposed to COVID-19.
Earlier on Thursday, a panel of CDC advisers said boosters should only be offered to people 65 and older, nursing home residents and those ages 50 to 64 who have risky underlying health problems. The extra dose would be given once they are at least six months past their last Pfizer shot.
However, Walensky decided to make one recommendation that the panel had rejected – covering frontline workers.
So she put that recommendation back in, noting that such a move aligns with a FDA booster authorization decision earlier this week.
The panel had offered the option of a booster for those ages 18 to 49 who have chronic health problems and want one. But the advisers refused to go further and open boosters to otherwise healthy frontline health care workers who aren't at risk of severe illness but want to avoid even a mild infection.
But, several hours after the panel adjourned, Walensky put it back in.
"As CDC Director, it is my job to recognize where our actions can have the greatest impact," Walensky said in a statement late Thursday night. "At CDC, we are tasked with analyzing complex, often imperfect data to make concrete recommendations that optimize health. In a pandemic, even with uncertainty, we must take actions that we anticipate will do the greatest good."
Walensky's change was unusual. Traditionally, CDC directors accept the advisory committee's recommendations without making substantive changes.
"I do think essential workers need a boost so that we can keep food on the shelves, lights on, and hospital staffed," Tufts Medical Center epidemiologist Dr. Shira Doron told WBZ-TV. "We are having a problem maintaining our staffing and providing the essential services that we are supposed to provide because we have people who are fully vaccinated getting COVID, and it's almost always mild, but they have to go out for 10 days."
MODERNA AND JOHNSON & JOHNSON
The CDC advisers expressed concern over the millions of Americans who received Moderna or Johnson & Johnson shots early in the vaccine rollout. The government still hasn't considered boosters for those brands and has no data on whether it is safe or effective to mix-and-match and give those people a Pfizer shot.
About 26 million Americans got their last Pfizer dose at least six months ago, about half of whom are 65 or older. It's not clear how many more would meet the CDC panel's booster qualifications.
CDC data shows the vaccines still offer strong protection against serious illness for all ages, but there is a slight drop among the oldest adults. And immunity against milder infection appears to be waning months after people's initial immunization.
For most people, if you're not in a group recommended for a booster, "it's really because we think you're well-protected," said Dr. Matthew Daley of Kaiser Permanente Colorado.
(© Copyright 2021 CBS Broadcasting Inc. All Rights Reserved. The Associated Press contributed to this report.)