CBS News Live
CBS News Boston: Local News, Weather & More
CBS News Boston is your streaming home for breaking news, weather, traffic and sports for the Boston area and beyond. Watch 24/7.
Watch CBS News

A truck landed on top of several parked cars after it crashed into a building in Brockton on Friday morning.

Believe it or not, according to data from the Tax Foundation, Massachusetts ranks better than 13 other states.

A person self-immolated at a park across from the courthouse where former President Donald Trump's New York criminal trial is taking place.

Businesses near TD Garden in Boston are preparing for a jam-packed weekend, with the Bruins and Celtics both starting the playoffs.

Boston marked 109 years since the Armenian Genocide began with a commemoration at the State House, where a 110-year-old genocide survivor was also honored.

Here we go again with another Celtics-Heat playoff series, which will tip off Sunday in Boston.

The Bruins and the Maple Leafs are once again taking their rivalry to the NHL Playoffs.

April 20, 2024 is Record Store Day. See which record shops in Massachusetts are participating.

Students at Harvard University and Northeastern University are taking classes in Taylor Swift that introduce them to poetry and improve their writing skills.

A look at the timeline of events in Karen Read's high-profile Massachusetts murder trial as she is charged with killing Boston police officer John O'Keefe.

Aidan Kearney, the Massachusetts blogger who goes by the name "Turtleboy," has become a key figure in the Karen Read murder trial.

Karen Read is on trial, charged with killing her boyfriend, Boston police officer John O'Keefe. Here's what to know about the trial.

Caroline Clim is the founder of her school's Morgan's Message Program, which is dedicated to helping student athletes with their mental health.

Taylor Swift dropped her new album, The Tortured Poets Department, early Friday morning and among those staying up were students in a Harvard University class dedicated to her music. WBZ TV's Paul Burton reports.

For WBZ’s Question Everything series, we asked whether our state still lives up to the old nickname Taxachusetts, and looked into how much of the Bay State’s bad tax reputation is myth versus reality. WBZ-TV's Christina Hager reports.

Players on the team said Tilly Danoff has taught them about finding joy and getting through tough times. WBZ TV's Tiffany Chan reports.
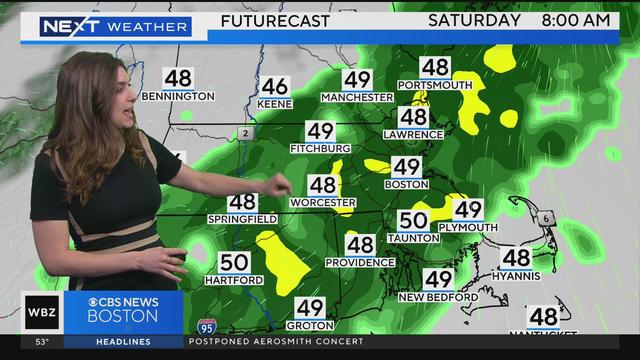
Alyssa Andrews has the latest forecast.

State contracts obtained by the I-Team show taxpayers are paying tens of millions of dollars to hotels for rooms and food.

A fourth-grade teacher at an elementary school in Cambridge is facing child rape charges.

The CDC estimates the U.S. could reach 300 measles cases in 2024 — more than the recent peak two years ago.

A new study finds that exercise may help reverse aging by reducing the buildup of fat.

Here we go again with another Celtics-Heat playoff series, which will tip off Sunday in Boston.

Brayan Bello allowed one hit in six innings, Rob Refsnyder hit his first home run of the season and the Boston Red Sox breezed past the struggling Pittsburgh Pirates 8-1 on Friday night.

The Celtics and the Miami Heat are set to square off for the third straight postseason, with Boston looking to avenge last year's loss in the Eastern Conference Finals as it begins its quest for an 18th NBA championship.

The Boston Bruins may have found just the solution to their playoff slump: A first-round matchup with the Toronto Maple Leafs.

The Red Sox have called up reliever Cam Booser, who will get his first taste of the majors at the age of 31.

The Bruins and the Maple Leafs are once again taking their rivalry to the NHL Playoffs.

The Red Sox are back to .500 after an embarrassing 3-7 homestand.

Will it be Linus Ullmark or Jeremy Swayman in net when the Bruins begin their first-round series against the Toronto Maple Leafs on Saturday night at TD Garden?

The injury bug keeps biting the Boston Red Sox.

Believe it or not, according to data from the Tax Foundation, Massachusetts ranks better than 13 other states.

When WBZ-TV showed Boston Red Sox fans pictures of current players, few could name any of them.
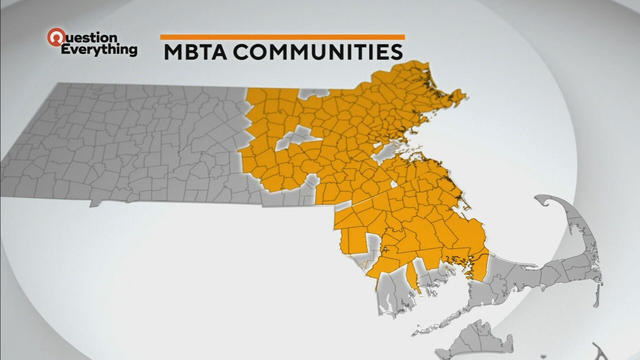
A law designed to ease the housing crunch in Massachusetts is turning into a battle between the state and some communities.

In Falmouth, just down the road from the ferry that heads to Martha's Vineyard, is an aquarium that happens to be a piece of history on Cape Cod.

Ice cream lovers are coming from all over to Holy Cow Ice Cream Cafe for their homemade ice cream and unique flavors.

A Somerville business is bringing pinball players together as they get their nostalgia fix.

The CDC estimates the U.S. could reach 300 measles cases in 2024 — more than the recent peak two years ago.

A new study finds that exercise may help reverse aging by reducing the buildup of fat.

Health officials are warning consumers not to consume Infinite Herbs basil sold at some Trader Joe's and Dierberg's stores after 12 people were sickened.

Mass General Hospital will add 94 inpatient beds by 2027.

Researchers in Singapore looked at whether or not plant-based meat alternatives provided the same health benefits as real meat.

State contracts obtained by the I-Team show taxpayers are paying tens of millions of dollars to hotels for rooms and food.

A fourth-grade teacher at an elementary school in Cambridge is facing child rape charges.

William Foley Jr., a five-time drunk driver who lost his license after a fatal crash in 2001, is back behind bars after an I-Team investigation.

The IRS Criminal Investigation unit helps go after violent criminals, including some right here in Boston.

A pet DNA testing company out of New Hampshire linked dog breeds to a human sample – twice.

According to ADL's annual Audit of Antisemitic Incidents, Massachusetts had the fifth most incidents in the country last year, up a shocking 189 percent over 2022.

"It's the golden goose," said Rooney of Boston's commercial property taxes, which provides a large percentage of the city's annual revenue.

MBTA General Manager Phil Eng gets high marks for improving the T's management and culture, but there's a lot of red ink that needs to be dealt with, says WBZ-TV's Jon Keller.

As Beacon Hill struggles to deal with a surge of homeless migrants, Governor Maura Healey finds herself dealing with another surge -- of political outrage over reports of migrant crime.

A new study of driving automation technology says 11 of 14 systems performed poorly under optimal road conditions.

April 20, 2024 is Record Store Day. See which record shops in Massachusetts are participating.

Boston Dynamics' latest robot is ready to "exceed human capabilities," the company says.

Rising costs are forcing small businesses located on Boston's Massachusetts Avenue to close.

Boston-based box wine brand Alileo teamed up with Caffe Nero to launch a coffee and wine experience in Massachusetts.

Today is Patriots' Day in Massachusetts. Here's what to know about the celebrations and what's open and closed for the holiday.

The 2024 cicada broods will not be emerging in Massachusetts - but the buzzing bugs are set to appear here in 2025.

A pair of vultures thought to be "actively dying" actually had too much to drink, wildlife rescuers in Connecticut say.

A fluffy pile of golden retrievers took over the Boston Common in memory of Spencer the marathon dog.

The thick fog made for an eerie sight as the Mayflower II crossed the Cape Cod Canal.

A piece of decades-old military equipment washed up on a Cape Cod beach last week.

The Vermont Country Store has since become an iconic destination for locals and tourists alike.

Creating an inviting environment for the community to meet, enjoy and participate in the arts

For over 85 years, Weston Theater Company has been creating engaging, entertaining, and inspiring theater.

Surrounded by mountains, Kimpton Taconic is a boutique hotel offering both classic and highly curated experiences.

Learn how the strategic use of windows and harnessing the power of daylight can create space and comfort within a home.

Students at Boston University took to the streets Friday, protesting violence in the Middle East, claiming school officials are staying silent ignoring the needs of Palestinian students.

Taylor Swift broke her own records, Spotify said, and now owns the record for the top three most-streamed albums in a single day.

Believe it or not, according to data from the Tax Foundation, Massachusetts ranks better than 13 other states.

Students at Harvard University and Northeastern University are taking classes in Taylor Swift that introduce them to poetry and improve their writing skills.

Businesses near TD Garden in Boston are preparing for a jam-packed weekend, with the Bruins and Celtics both starting the playoffs.

Here we go again with another Celtics-Heat playoff series, which will tip off Sunday in Boston.

Brayan Bello allowed one hit in six innings, Rob Refsnyder hit his first home run of the season and the Boston Red Sox breezed past the struggling Pittsburgh Pirates 8-1 on Friday night.

The Celtics and the Miami Heat are set to square off for the third straight postseason, with Boston looking to avenge last year's loss in the Eastern Conference Finals as it begins its quest for an 18th NBA championship.

The Boston Bruins may have found just the solution to their playoff slump: A first-round matchup with the Toronto Maple Leafs.

The Red Sox have called up reliever Cam Booser, who will get his first taste of the majors at the age of 31.