'Downside Is Zero': Doctor Who Nearly Died From COVID-19 Credits Convalescent Plasma For Recovery
WORCESTER (CBS) - UMass Memorial gastroenterologist Dr. Savant Mehta battled COVID-19 on a ventilator for two weeks. His wife, Sonia, watched in agony as her husband went from physician to helpless patient.
"There was like no hope," Sonia said.
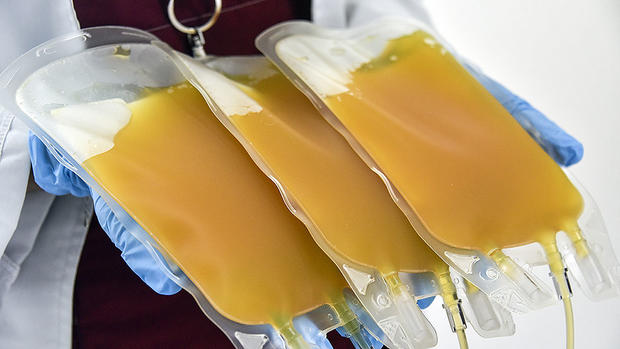
As a last resort to save Mehta's life, doctors at UMass Memorial Medical Center in Worcester used two transfusions of convalescent plasma from recovered COVID-19 patients.
"I think three days later [he was] off the ventilator. They kept him three days just to keep an eye on him but he was improving, marked improvement," Sonia said.
Over the weekend, the Food and Drug Administration authorized the emergency use of convalescent plasma to treat COVID-19 in hospitalized patients. The idea is that antibodies from recovered patients can transfer to very ill patients through plasma, allowing them to fight off the virus.
Some scientists remain skeptical of the benefits of plasma since no randomized clinical trial data is available yet.
More than 100 critically ill COVID-19 patients have been treated with convalescent plasma through a UMass Medical School clinical trial led by Dr. Jonathan Gerber, the medical director of the UMass Cancer Center. Despite the lack of data, Gerber says the pros appear to outweigh the cons.
"We've seen a number of successes. We now have treated 146 patients and over 90 of those have recovered and are out of the hospital," Gerber said.
Mehta credits the plasma with saving his life. "The downside is zero and the upside is very positive, especially if you're in a life threatening situation. I think most people would say, yes, I'll take it," he said.